Synthesis and folding behaviour of poly(p-phenylene vinylene)-based β-sheet polychromophores†
Abstract
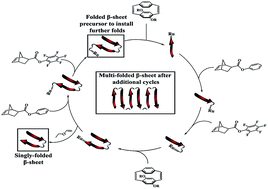
This contribution describes the design and synthesis of β-sheet-mimicking synthetic polymers comprising distinct poly(p-phenylene vinylene) (PPV) and poly(norbornene) (PNB) backbones with multiple turns. The rod–coil–coil–rod tetrablock copolymers, synthesized using ring-opening metathesis polymerization (ROMP) and featuring orthogonal face-to-face π–π stacking and phenyl/perfluorophenyl interactions, show persistent folding both in bulk and at the single molecule level, irrespective of the number of β-turns. Single molecule polarization studies reveal that the copolymers are more anisotropic than the corresponding homopolymers. Examination of the spectral signatures of the single molecules shows a dominant emissive chromophore in the linked materials compared to the homopolymer. The lack of significant spectral changes of the folded materials along with the existence of a dominant emission spectrum supports the proposed structure of well-aligned, minimally-interacting chromophores. Utilization of this reliably folding, phenyl/perfluorophenyl functionality could provide an extremely useful tool in future functional materials design.


 Please wait while we load your content...
Please wait while we load your content...