Direct N–O bond formation via oxidation of amines with benzoyl peroxide†
Abstract
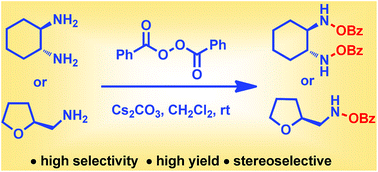
Herein, we report a general and efficient method for direct N–O bond formation without undesirable C–N bond (amide) formation starting from commercially available amines and benzoyl peroxide. The oxidation of 1,2-diamines to furnish bis-(benzoyloxy)-1,2-diamines is reported for the first time. We found that a significant amount of water (BPO : water = 3 : 1) in combination with Cs2CO3 is necessary to achieve high selectivity and yield. The reaction conditions are applicable to a wide range of 1,2-diamine and 1,2-disubstituted-1,2-diamine substrates. Additionally this method is highly applicable to primary and secondary amines. Further, the present method can access chiral bis-hydroxamic acids and bis-hydroxyl amines in just two steps from 1,2-diamines. The reaction conditions are simple, mild and inert atmosphere free. The synthetic potential of this methodology is further demonstrated in the short synthesis of a chiral BHA ligand.


 Please wait while we load your content...
Please wait while we load your content...