Building blocks for recognition-encoded oligoesters that form H-bonded duplexes†
Abstract
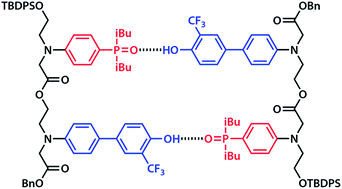
Competition from intramolecular folding is a major challenge in the design of synthetic oligomers that form intermolecular duplexes in a sequence-selective manner. One strategy is to use very rigid backbones that prevent folding, but this design can prejudice duplex formation if the geometry is not exactly right. The alternative approach found in nucleic acids is to use bases (or recognition units) that have different dimensions. A long-short base-pairing scheme makes folding geometrically difficult and is compatible with the flexible backbones that are required to guarantee duplex formation. A monomer building block equipped with a long hydrogen bond donor (phenol, D) recognition unit and a monomer building block equipped with a short hydrogen bond acceptor (phosphine oxide, A) recognition unit were prepared with differentially protected alcohol and carboxylic acid groups. These compounds were used to synthesise the homo and hetero-sequence 2-mers AA, DD and AD. 19F and 31P NMR experiments were used to characterize the assembly properties of these compounds in toluene solution. AA and DD form a stable doubly-hydrogen-bonded duplex with an effective molarity of 20 mM for formation of the second intramolecular hydrogen bond. AD forms a duplex of similar stability. There is no evidence of intramolecular folding in the monomeric state of this compound, which shows that the long-short base-pairing scheme is effective. The ester coupling chemistry used here is an attractive method for the synthesis of long oligomers, and the properties of the 2-mers indicate that this molecular architecture should give longer mixed sequence oligomers that show high fidelity sequence-selective duplex formation.


 Please wait while we load your content...
Please wait while we load your content...