Nickel-catalyzed cyanation of aryl halides and triflates using acetonitrile via C–CN bond cleavage assisted by 1,4-bis(trimethylsilyl)-2,3,5,6-tetramethyl-1,4-dihydropyrazine†
Abstract
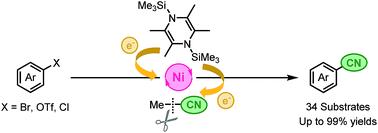
We developed a non-toxic cyanation reaction of various aryl halides and triflates in acetonitrile using a catalyst system of [Ni(MeCN)6](BF4)2, 1,10-phenanthroline, and 1,4-bis(trimethylsilyl)-2,3,5,6-tetramethyl-1,4-dihydropyrazine (Si–Me4-DHP). Si–Me4-DHP was found to function as a reductant for generating nickel(0) species and a silylation reagent to achieve the catalytic cyanation via C–CN bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...