Testosterone meets albumin – the molecular mechanism of sex hormone transport by serum albumins†
Abstract
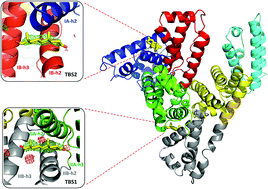
Serum albumin is the most abundant protein in mammalian blood plasma and is responsible for the transport of metals, drugs, and various metabolites, including hormones. We report the first albumin structure in complex with testosterone, the primary male sex hormone. Testosterone is bound in two sites, neither of which overlaps with the previously suggested Sudlow site I. We determined the binding constant of testosterone to equine and human albumins by two different methods: tryptophan fluorescence quenching and ultrafast affinity extraction. The binding studies and similarities between residues comprising the binding sites on serum albumins suggest that testosterone binds to the same sites on both proteins. Our comparative analysis of albumin complexes with hormones, drugs, and other biologically relevant compounds strongly suggests interference between a number of compounds present in blood and testosterone transport by serum albumin. We discuss a possible link between our findings and some phenomena observed in human patients, such as low testosterone levels in diabetic patients.


 Please wait while we load your content...
Please wait while we load your content...