Simple electrochemical reduction of nitrones to amines†
Abstract
The use of electricity allows the reduction of nitrones containing aromatic and heteroaromatic rings to the corresponding amines. The main advantage of this protocol relies on the fact that only electrons are needed, avoiding the use of different chemicals and harsh procedures, something that reinforces some green aspects. The conversion tolerates different moieties and a large variety of functional groups. In addition, this electrolysis can be performed on a simple undivided beaker-type cell with constant current conditions.
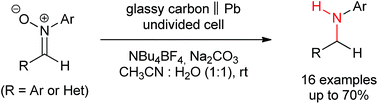


 Please wait while we load your content...
Please wait while we load your content...