A metal-catalyzed new approach for α-alkynylation of cyclic amines†
Abstract
The first catalytic α-alkynylation of cyclic amines with the help of the N-propargylic group to afford 2-(1-alkynyl) N-allylic cyclic amines with an exclusive E-stereoselectivity for the in situ formed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond has been realized. Based on mechanistic studies, it is proven that the reaction proceeds through metal-mediated anti-1,5-hydride transfer forming an iminonium intermediate, which accepts the addition of the in situ generated 1-alkynyl metal species. The synthetic application has also been demonstrated.
C bond has been realized. Based on mechanistic studies, it is proven that the reaction proceeds through metal-mediated anti-1,5-hydride transfer forming an iminonium intermediate, which accepts the addition of the in situ generated 1-alkynyl metal species. The synthetic application has also been demonstrated.
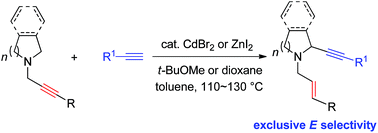


 Please wait while we load your content...
Please wait while we load your content...