Diarylmethane synthesis through Re2O7-catalyzed bimolecular dehydrative Friedel–Crafts reactions†
Abstract
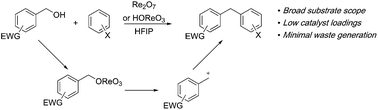
This manuscript describes the application of Re2O7 to the syntheses of diarylmethanes from benzylic alcohols through solvolysis followed by Friedel–Crafts alkylation. The reactions are characterized by broad substrate scope, low catalyst loadings, high chemical yields, and minimal waste generation. The intermediate perrhenate esters are superior leaving groups to chlorides and bromides in these reactions. The polarity and water sequestering capacity of hexafluoroisopropyl alcohol are critical to the success of these processes. Re2O7 is a precatalyst for HOReO3, which serves as a less costly and easily handled promoter for these reactions. Oxorhenium catalysts selectively activate alcohols in the presence of similarly substituted acetates, indicating a unique chemoselectivity and mechanism in comparison to Brønsted acid catalysis.


 Please wait while we load your content...
Please wait while we load your content...