From an Fe2P3 complex to FeP nanoparticles as efficient electrocatalysts for water-splitting†
Abstract
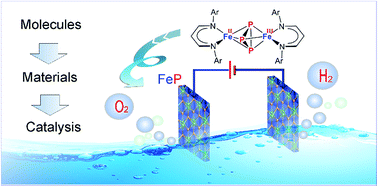
In large-scale, hydrogen production from water-splitting represents the most promising solution for a clean, recyclable, and low-cost energy source. The realization of viable technological solutions requires suitable efficient electrochemical catalysts with low overpotentials and long-term stability for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) based on cheap and nontoxic materials. Herein, we present a unique molecular approach to monodispersed, ultra-small, and superiorly active iron phosphide (FeP) electrocatalysts for bifunctional OER, HER, and overall water-splitting. They result from transformation of a molecular iron phosphide precursor, containing a [Fe2P3] core with mixed-valence FeIIFeIII sites bridged by an asymmetric cyclo-P(2+1)3− ligand. The as-synthesized FeP nanoparticles act as long-lasting electrocatalysts for OER and HER with low overpotential and high current densities that render them one of the best-performing electrocatalysts hitherto known. The fabricated alkaline electrolyzer delivered low cell voltage with durability over weeks, representing an attractive catalyst for large-scale water-splitting technologies.
- This article is part of the themed collections: Most popular 2018-2019 energy articles and Most popular 2018-2019 nanoscience articles


 Please wait while we load your content...
Please wait while we load your content...