Anion identification using silsesquioxane cages†
Abstract
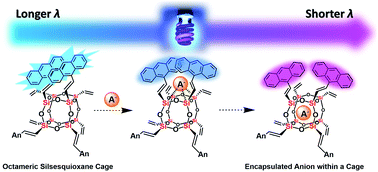
Anthracene-conjugated octameric silsesquioxane (AnSQ) cages, prepared via Heck coupling between octavinylsilsesquioxane (OVS) and 9-bromoanthracene, thermodynamically display intramolecular excimer emissions. More importantly, these hosts are sensitive to each anionic guest, thereby resulting in change of anthracene excimer formation, displaying the solvent-dependent fluorescence and allowing us to distinguish up to four ions such as F−, OH−, CN− and PO43− by fluorescence spectroscopy. Depending on the solvent polarity, for example, both F− and CN− quenched the fluorescence emission intensity in THF, but only F− could enhance the fluorescence in all other solvents. The presence of PO43− results in fluorescence enhancements in high polarity solvents such as DMSO, DMF, and acetone, while OH− induces enhancements only in low polarity solvents (e.g. DCM and toluene). A picture of the anion recognizing ability of AnSQ was obtained through principal component analysis (PCA) with NMR and FTIR confirming the presence of host–guest interactions. Computational modeling studies demonstrate the conformation of host–guest complexation and also the change of excimer formation. Detection of F−, CN− and OH− by AnSQ hosts in THF is noticeable with the naked eye, as indicated by strong color changes arising from charge transfer complex formation upon anion addition.


 Please wait while we load your content...
Please wait while we load your content...