A long-lived peptide-conjugated iridium(iii) complex as a luminescent probe and inhibitor of the cell migration mediator, formyl peptide receptor 2†
Abstract
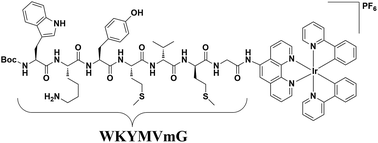
Formyl peptide receptors play important biological and therapeutic roles in wound repair and inflammatory diseases. In this work, we present a luminescent iridium(III) complex (6) conjugated with the peptide agonist WKYMVm as a luminescent formyl peptide receptor 2 (FPR2) imaging probe in living cells. Complex 6 displayed ideal cell imaging characteristics, high photostability and low cytotoxicity. Competition assays with a known FPR2 antagonist, WRW4, and siRNA knockdown experiments both revealed that complex 6 selectively targeted FPR2 in living HUVEC cells. Moreover, complex 6 regulated FPR2 signalling in HUVEC cells as shown using a mechanical scratch assay. Finally, complex 6 reduced epithelial cell migration capacity and inhibited lipoxin A4 (LXA4)-triggered cell migration in HUVEC cells, demonstrating the ability of this complex to inhibit FPR2 in living cells. To our knowledge, this is the first long-lived probe for imaging FPR2 in living cells.
- This article is part of the themed collection: Most popular 2018-2019 analytical chemistry articles


 Please wait while we load your content...
Please wait while we load your content...