Electrophile-promoted Fe-to-N2 hydride migration in highly reduced Fe(N2)(H) complexes†
Abstract
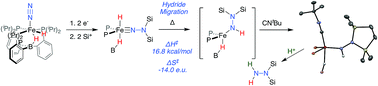
One of the emerging challenges associated with developing robust synthetic nitrogen fixation catalysts is the competitive formation of hydride species that can play a role in catalyst deactivation or lead to undesired hydrogen evolution reactivity (HER). It is hence desirable to devise synthetic systems where metal hydrides can migrate directly to coordinated N2 in reductive N–H bond-forming steps, thereby enabling productive incorporation into desired reduced N2-products. Relevant examples of this type of reactivity in synthetic model systems are limited. In this manuscript we describe the migration of an iron hydride (Fe-H) to Nα of a disilylhydrazido(2-) ligand (Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNR2) derived from N2via double-silylation in a preceding step. This is an uncommon reactivity pattern in general; well-characterized examples of hydride/alkyl migrations to metal heteroatom bonds (e.g., (R)M
NNR2) derived from N2via double-silylation in a preceding step. This is an uncommon reactivity pattern in general; well-characterized examples of hydride/alkyl migrations to metal heteroatom bonds (e.g., (R)M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR′ → M–N(R)R′) are very rare. Mechanistic data establish the Fe-to-Nα hydride migration to be intramolecular. The resulting disilylhydrazido(1-) intermediate can be isolated by trapping with CNtBu, and the disilylhydrazine product can then be liberated upon treatment with an additional acid equivalent, demonstrating the net incorporation of an Fe-H equivalent into an N-fixed product.
NR′ → M–N(R)R′) are very rare. Mechanistic data establish the Fe-to-Nα hydride migration to be intramolecular. The resulting disilylhydrazido(1-) intermediate can be isolated by trapping with CNtBu, and the disilylhydrazine product can then be liberated upon treatment with an additional acid equivalent, demonstrating the net incorporation of an Fe-H equivalent into an N-fixed product.


 Please wait while we load your content...
Please wait while we load your content...