Photocatalytic Barbier reaction – visible-light induced allylation and benzylation of aldehydes and ketones†
Abstract
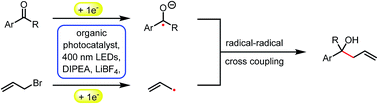
We report a photocatalytic version of the Barbier type reaction using readily available allyl or benzyl bromides and aromatic aldehydes or ketones as starting materials to generate allylic or benzylic alcohols. The reaction proceeds at room temperature under visible light irradiation with the organic dye 3,7-di(4-biphenyl)1-naphthalene-10-phenoxazine as a photocatalyst and DIPEA as sacrificial electron donor. The proposed cross-coupling mechanism of a ketyl- and an allyl or benzyl radical is supported by spectroscopic investigations and cyclic voltammetry measurements.


 Please wait while we load your content...
Please wait while we load your content...