Let there be light: stability of palmitic acid monolayers at the air/salt water interface in the presence and absence of simulated solar light and a photosensitizer†
Abstract
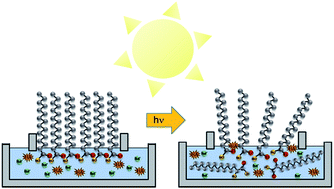
Long-chain fatty acid monolayers are known surfactants present at air/water interfaces. However, little is known about the stability of these long-chain fatty acid monolayers in the presence of solar radiation. Here we have investigated, for the first time, the stability of palmitic acid monolayers on salt water interfaces in the presence and absence of simulated solar light with and without a photosensitizer in the underlying salt subphase. Using surface sensitive probes to measure changes in the properties of these monolayers upon irradiation, we found that the monolayers become less stable in the presence of light and a photosensitizer, in this case humic acid, in the salt solution. The presence of the photosensitizer is essential in significantly reducing the stability of the monolayer upon irradiation. The mechanism for this loss of stability is due to interfacial photochemistry involving electronically excited humic acid and molecular oxygen reacting with palmitic acid at the interface to form more oxygenated and less surface-active species. These oxygenated species can then more readily partition into the underlying solution.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...