(Salen)Mn(iii)-catalyzed chemoselective acylazidation of olefins†
Abstract
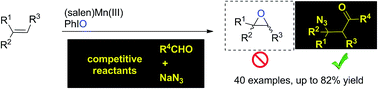
We describe a (salen)Mn(III)-catalyzed three-component reaction of aldehydes, olefins, and sodium azide for the installation of two useful groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N3) into the double bond. Traditionally, (salen)Mn(III) in conjunction with iodosobenzene is a classical catalysis system for epoxidation of olefins. Owing to the highly competitive oxygenation approaches, it is a true challenge to establish a distinct strategy for the exploration of new olefin transformations based on this (salen)Mn(III) catalysis system. Herein, the key to this (salen)Mn(III)-catalyzed acylazidation of olefins was the rational application of the distinct reactivity of oxomanganese(V) species which is capable of abstracting a hydrogen atom from a substrate C–H bond. This chemoselective reaction occurred in a precisely designed reaction sequence and tolerates complex molecular structures.
O and N3) into the double bond. Traditionally, (salen)Mn(III) in conjunction with iodosobenzene is a classical catalysis system for epoxidation of olefins. Owing to the highly competitive oxygenation approaches, it is a true challenge to establish a distinct strategy for the exploration of new olefin transformations based on this (salen)Mn(III) catalysis system. Herein, the key to this (salen)Mn(III)-catalyzed acylazidation of olefins was the rational application of the distinct reactivity of oxomanganese(V) species which is capable of abstracting a hydrogen atom from a substrate C–H bond. This chemoselective reaction occurred in a precisely designed reaction sequence and tolerates complex molecular structures.


 Please wait while we load your content...
Please wait while we load your content...