Reversible coordination of N2 and H2 to a homoleptic S = 1/2 Fe(i) diphosphine complex in solution and the solid state†
Abstract
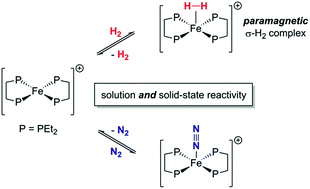
The synthesis and characterisation of the S = 1/2 Fe(I) complex [Fe(depe)2]+[BArF4]− ([1]+[BArF4]−), and the facile reversible binding of N2 and H2 in both solution and the solid state to form the adducts [1·N2]+ and [1·H2]+, are reported. Coordination of N2 in THF is thermodynamically favourable under ambient conditions (1 atm; ΔG298 = −4.9(1) kcal mol−1), while heterogenous binding is more favourable for H2 than N2 by a factor of ∼300. [1·H2]+[BArF4]− represents a rare example of a well-defined, open-shell, non-classical dihydrogen complex, as corroborated by ESR spectroscopy. The rapid exchange between N2 and H2 coordination under ambient conditions is unique for a paramagnetic Fe complex.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...