Thermodynamic versus kinetic control in substituent redistribution reactions of silylium ions steered by the counteranion†
Abstract
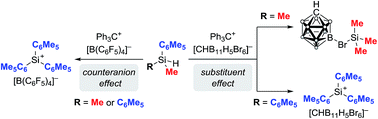
An in-depth experimental and theoretical study of the substituent exchange reaction of silylium ions is presented. Apart from the substitution pattern at the silicon atom, the selectivity of this process is predominantly influenced by the counteranion, which is introduced with the trityl salt in the silylium ion generation. In contrast to Müller's protocol for the synthesis of triarylsilylium ions under kinetic control, the use of Reed's carborane anions leads to contact ion pairs, allowing selective formation of trialkylsilylium ions under thermodynamic control. DFT calculations finally revealed an unexpected mechanism for the rate-determining alkyl exchange step, which is initiated by an unusual 1,2-silyl migration in the intermediate ipso-disilylated arenium ion. The resulting ortho-disilylated arenium ion can then undergo an alkyl transfer via a low-barrier five-centered transition state.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...