Oxidative dehalogenation and denitration by a flavin-dependent monooxygenase is controlled by substrate deprotonation†
Abstract
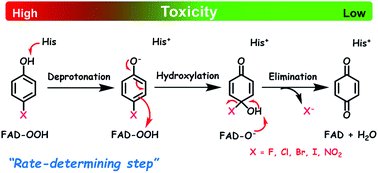
Enzymes that are capable of detoxifying halogenated phenols (HPs) and nitrophenols (NPs) are valuable for bioremediation and waste biorefining. HadA monooxygenase was found to perform dual functions of oxidative dehalogenation (hydroxylation plus halide elimination) and denitration (hydroxylation plus nitro elimination). Rate constants associated with individual steps of HadA reactions with phenol, halogenated phenols and nitrophenols were measured using combined transient kinetic approaches of stopped-flow absorbance/fluorescence and rapid-quench flow techniques. Density functional theory was used to calculate the thermodynamic and electronic parameters associated with hydroxylation and group elimination steps. These parameters were correlated with the rate constants of hydroxylation, group elimination, and overall product formation to identify factors controlling individual steps. The results indicated that the hydroxylation rate constant is higher when the pKa of the phenolic group is lower, i.e. it is more easily deprotonated, but not higher when the energy gap between the ELUMO of the C4a-hydroperoxy-FAD intermediate and the EHOMO of the phenolate substrate is lower. These data suggest that the substrate deprotonation has a higher energy barrier than the –OH transfer, and thus controls the hydroxylation step. For the group elimination, the process is controlled by the ability of the C–X bond to break. For the overall product formation (hydroxylation and group elimination combined), this analysis showed that the rate constant of product formation is dependent on the pKa value of the substrate, indicating that the overall reaction is controlled by substrate deprotonation. This step also likely has the highest energy barrier and thus controls the overall process of oxidative dehalogenation and denitration by HadA. This report is the first to identify a key mechanistic factor controlling the enzymatic processes of oxidative dehalogenation and denitration.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...