A paramedic treatment for modeling explicitly solvated chemical reaction mechanisms†
Abstract
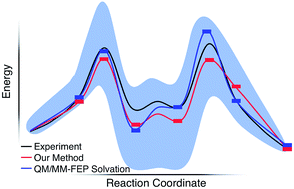
We report a static quantum chemistry modeling treatment to study how solvent molecules affect chemical reaction mechanisms without dynamics simulations. This modeling scheme uses a global optimization procedure to identify low energy intermediate states with different numbers of explicit solvent molecules and then the growing string method to locate sequential transition states along a reaction pathway. Testing this approach on the acid-catalyzed Morita–Baylis–Hillman (MBH) reaction in methanol, we found a reaction mechanism that is consistent with both recent experiments and computationally intensive dynamics simulations with explicit solvation. In doing so, we explain unphysical pitfalls that obfuscate computational modeling that uses microsolvated reaction intermediates. This new paramedic approach can promisingly capture essential physical chemistry of the complicated and multistep MBH reaction mechanism, and the energy profiles found with this model appear reasonably insensitive to the level of theory used for energy calculations. Thus, it should be a useful and computationally cost-effective approach for modeling solvent mediated reaction mechanisms when dynamics simulations are not possible.
- This article is part of the themed collection: The ChemRxiv Collection


 Please wait while we load your content...
Please wait while we load your content...