Curved π-conjugated corannulene dimer diradicaloids†
Abstract
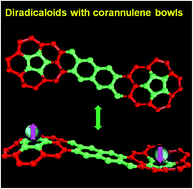
So far, most reported open-shell singlet diradicaloids are based on planar π-conjugated molecules. Herein, we report the bridged corannulene dimer diradicaloids, Cor-D1 and Cor-D2, both showing a three-dimensional curved π-conjugated structure. Cor-D1 has a small diradical character (y0 = 5.4%) and behaves more like a closed-shell quinoidal compound at room temperature, while Cor-D2 is a typical open-shell diradicaloid with a larger diradical character (y0 = 16.9%). Both compounds exhibited paramagnetic activity at elevated temperatures, with a singlet–triplet energy gap (ΔES–T) of −8.4 and −3.0 kcal mol−1, respectively. X-ray crystallographic analysis revealed that both molecules have a dumbbell-shaped geometry, with the two terminal corannulene bowls bent to opposite directions. The spin is largely delocalized onto the two bowls in Cor-D2 and there are multiple [CH⋯π] interactions between the neighboring bowls. Chemical oxidation/reduction to their respective dications/dianions results in global aromaticity with [4n + 2] π-electrons delocalized through the periphery of the whole framework.


 Please wait while we load your content...
Please wait while we load your content...