Facile access to deep red/near-infrared emissive AIEgens for efficient non-doped OLEDs†
Abstract
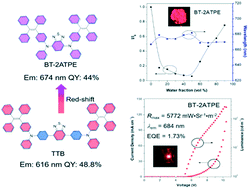
Notwithstanding the huge demand in bio-imaging and optoelectronics, the construction of highly emissive deep red/near infrared (DR/NIR) organic luminogens is still a big challenge because a narrow energy gap generally leads to low photoluminescence quantum yield. It is even more difficult to afford DR/NIR emitters in the solid state due to the aggregation caused quenching (ACQ) effect. In this work, we found that the direct attachment of a tetraphenylethylene substituted arylamine to the electron accepting 2,1,3-benzothiadiazole produces DR/NIR AIE luminogens with bright emission facilely and efficiently. And the emission wavelengths could be tuned from the red to the DR/NIR region by regulating the variety of the substituents. The long emission wavelength and high photoluminescence quantum yield of these AIEgens are ascribed to the effective intramolecular charge transfer and the suppressed intramolecular motion. Furthermore, non-doped OLEDs based on one of the AIEgens showed an EL emission at 684 nm with a large radiance of 5772 mW Sr−1 m−2 and an impressive external quantum efficiency (EQE) of 1.73%.


 Please wait while we load your content...
Please wait while we load your content...