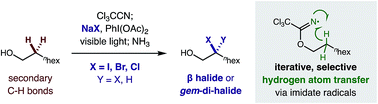
β C–H di-halogenation via iterative hydrogen atom transfer†
Abstract
A radical relay strategy for mono- and di-halogenation (iodination, bromination, and chlorination) of sp3 C–H bonds has been developed. This first example of β C–H di-halogenation is achieved through sequential C–H abstraction by iterative, hydrogen atom transfer (HAT). A double C–H functionalization is enabled by in situ generated imidate radicals, which facilitate selective N˙ to C˙ radical translocation and tunable C–X termination. The versatile, geminal di-iodide products are further elaborated to β ketones and vinyl iodides. Mechanistic experiments explain the unique di-functionalization selectivity of this iterative HAT pathway, wherein the second C–H iodination is twice as fast as the first.


 Please wait while we load your content...
Please wait while we load your content...