A DFT-based mechanistic proposal for the light-driven insertion of dioxygen into Pt(ii)–C bonds†
Abstract
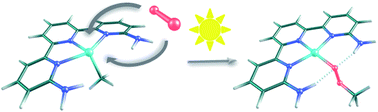
The photocatalyzed insertion of dioxygen into the Pt(II)–methyl bond in terpyridine platinum complexes has been shown to proceed efficiently, but its mechanism remains a challenge. In particular, there are serious counter-intuitive differences in the reactivity of structurally similar complexes. M06 calculations in solvent with a valence double-ζ basis set supplemented by polarization and diffusion shells (benchmarked against ωB97x-D calculations with a larger basis set) are able to provide a satisfactory mechanistic answer. The proposed mechanism starts with the absorption of a photon by the metal complex, which then evolves into a triplet state that reacts with the triplet dioxygen fragment. A variety of possible reaction paths have been identified, some leading to the methylperoxo product and others reverting to the reactants, and the validity of some of these paths has been confirmed by additional experiments. The balance between the barriers towards productive and unproductive paths reproduces the diverging experimental behavior of similar complexes and provides a general mechanistic picture for these processes.


 Please wait while we load your content...
Please wait while we load your content...