A highly site-selective radical sp3 C–H amination of azaheterocycles†
Abstract
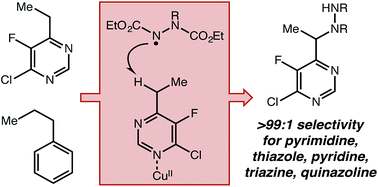
This report describes the development of a novel C–H amination strategy using both a Cu(II) Lewis acid and an organic hydrogen atom transfer catalyst to activate benzylic C–H bonds adjacent to aromatic N-heterocycles. This simple methodology demonstrates very high selectivity towards azaheterocycles without using exogenous directing groups and affords excellent site selectivity in substrates with more than one reactive position. A wide range of heterocyclic structures not compatible with previously reported catalytic systems have proven to be amenable to this approach. Mechanistic investigations strongly support a radical-mediated H-atom abstraction, which explains the observed contrast to known closed-shell Lewis acid catalyzed processes.


 Please wait while we load your content...
Please wait while we load your content...