Reactivity enhancement of a diphosphene by reversible N-heterocyclic carbene coordination†
Abstract
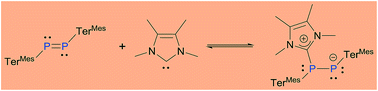
Diphosphene TerMesP = PTerMes (1; TerMes = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2) and NHCMe42 (NHCMe4 = 1,3,4,5-tetramethylimidazol-2-ylidene) exist in an equilibrium mixture with the NHCMe4-coordinated diphosphene 3. While uncoordinated 1 is inert to hydrolysis, the NHC adduct 3 readily undergoes hydrolysis to afford a phosphino-substituted phosphine oxide with the liberation of NHCMe4. On this basis, conditions suitable for the catalytic use of NHCMe4 were identified. Similarly, while the hydrogenation of free diphosphene 1 with H3N·BH3 is very slow, 3 reacts instantaneously with H3N·BH3 at room temperature to afford a dihydrodiphosphane.


 Please wait while we load your content...
Please wait while we load your content...