Metabolism and hydrophilicity of the polarised ‘Janus face’ all-cis tetrafluorocyclohexyl ring, a candidate motif for drug discovery†
Abstract
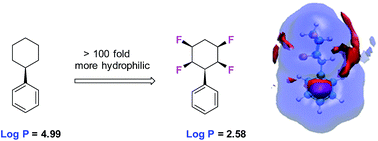
The metabolism and polarity of the all-cis tetra-fluorocyclohexane motif is explored in the context of its potential as a motif for inclusion in drug discovery programmes. Biotransformations of phenyl all-cis tetra-, tri- and di- fluoro cyclohexanes with the human metabolism model organism Cunninghamella elegans illustrates various hydroxylated products, but limited to benzylic hydroxylation for the phenyl all-cis tetrafluorocyclohexyl ring system. Evaluation of the lipophilicities (log P) indicates a significant and progressive increase in polarity with increasing fluorination on the cyclohexane ring system. Molecular dynamics simulations indicate that water associates much more closely with the hydrogen face of these Janus face cyclohexyl rings than the fluorine face owing to enhanced hydrogen bonding interactions with the polarised hydrogens and water.


 Please wait while we load your content...
Please wait while we load your content...