Non-competitive cyclic peptides for targeting enzyme–substrate complexes†
Abstract
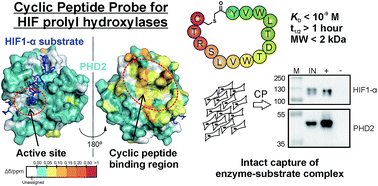
Affinity reagents are of central importance for selectively identifying proteins and investigating their interactions. We report on the development and use of cyclic peptides, identified by mRNA display-based RaPID methodology, that are selective for, and tight binders of, the human hypoxia inducible factor prolyl hydroxylases (PHDs) – enzymes crucial in hypoxia sensing. Biophysical analyses reveal the cyclic peptides to bind in a distinct site, away from the enzyme active site pocket, enabling conservation of substrate binding and catalysis. A biotinylated cyclic peptide captures not only the PHDs, but also their primary substrate hypoxia inducible factor HIF1-α. Our work highlights the potential for tight, non-active site binding cyclic peptides to act as promising affinity reagents for studying protein–protein interactions.


 Please wait while we load your content...
Please wait while we load your content...