Design, synthesis, and time-gated cell imaging of carbon-bridged triangulenium dyes with long fluorescence lifetime and red emission†
Abstract
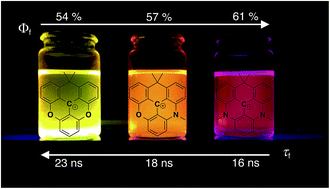
Time-resolved fluorescence offers many advantages over normal steady-state detection and becomes increasingly important in bioimaging. However, only very few fluorophores with emission in the visible range and fluorescence lifetimes above 5 ns are available. In this work, we prepare a series of new aza/oxa-triangulenium dyes where one of the usual oxa or aza bridges is replaced by an isopropyl bridge. This leads to a significant redshift of fluorescence with only moderate reductions of quantum yields and a unique long fluorescence lifetime. The fluorescence of the isopropyl bridged diazatriangulenium derivative CDATA+ is red-shifted by 50 nm (1400 cm−1) as compared to the oxygen-bridged DAOTA+ chromophore and has intense emission in the red region (600–700 nm) with a quantum yield of 61%, and a fluorescence lifetime of 15.8 ns in apolar solution. When the CDATA+ dye is used as cell stain, high photostability and efficient time-gated cell imaging is demonstrated.
- This article is part of the themed collections: Near-infrared (NIR) luminescent probes for bioimaging and biosensing and 2018 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...