A lesson for site-selective C–H functionalization on 2-pyridones: radical, organometallic, directing group and steric controls
Abstract
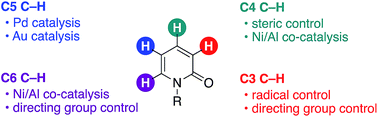
A 2-pyridone ring is a frequently occurring subunit in natural products, biologically active compounds, and pharmaceutical targets. Thus, the selective synthesis of substituted 2-pyridone derivatives through decoration and/or formation of pyridone rings has been one of the important longstanding subjects in organic synthetic chemistry. This minireview focuses on recent advances in site-selective C–H functionalization on 2-pyridone. The reported procedures are categorized according to the site selectivity that is achieved, and the substrate scope, limitations, mechanism, and controlling factors are briefly summarized.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...