Synthesis and kinetic resolution of substituted tetrahydroquinolines by lithiation then electrophilic quench†
Abstract
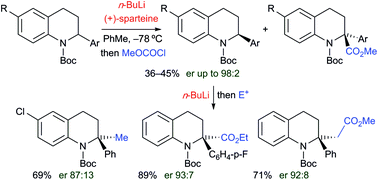
Treatment of N-Boc-2-aryl-1,2,3,4-tetrahydroquinolines with n-butyllithium in THF at −78 °C resulted in efficient lithiation at the 2-position and the organolithiums were trapped with a variety of electrophiles to give substituted products. Variable temperature NMR spectroscopy gave kinetic data that showed that the rate of tert-butoxycarbonyl (Boc) rotation was fast (ΔG‡ ≈ 45 kJ mol−1 at −78 °C) and in situ ReactIR spectroscopy showed fast lithiation at −78 °C. By carrying out the lithiation in the presence of the chiral ligand sparteine, kinetic resolutions with very high levels of enantioselectivity were achieved. The resulting enantioenriched N-Boc-2-aryltetrahydroquinolines were converted to 2,2-disubstituted products without significant loss in enantiopurity. Most electrophiles add at the 2-position and the chemistry provides a way to access tetrahydroquinolines that are fully substituted alpha to the nitrogen atom. Notably, either enantiomer of the 2,2-disubstituted tetrahydroquinolines can be obtained with high selectivity from the same enantiomer of the chiral ligand. Unusually, when methyl cyanoformate was used as the electrophile, substitution occurred in the ortho position of the aryl ring attached at C-2. This change in regioselectivity on changing the electrophile was probed by deuterium isotope studies and by DFT calculations which suggested that the binding of the cyanoformate altered the structure of the intermediate organolithium. Secondary amine products can be prepared by removing the Boc group with acid or by inducing the Boc group to rearrange to the 2-position in the presence of triethylborane and this carbonyl N-to-C rearrangement occurs with retention of configuration from the intermediate enantiomerically enriched organolithium species.


 Please wait while we load your content...
Please wait while we load your content...