Doping palladium with tellurium for the highly selective electrocatalytic reduction of aqueous CO2 to CO†
Abstract
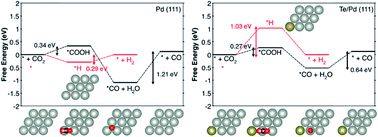
Designing highly selective and energy-efficient electrocatalysts to minimize the competitive hydrogen evolution reaction in the electrochemical reduction of aqueous CO2 remains a challenge. In this study, we report that doping Pd with a small amount of Te could selectively convert CO2 to CO with a low overpotential. The PdTe/few-layer graphene (FLG) catalyst with a Pd/Te molar ratio of 1 : 0.05 displayed a maximum CO faradaic efficiency of about 90% at −0.8 V (vs. a reversible hydrogen electrode, RHE), CO partial current density of 4.4 mA cm−2, and CO formation turnover frequency of 0.14 s−1 at −1.0 V (vs. a RHE), which were 3.7-, 4.3-, and 10-fold higher than those of a Pd/FLG catalyst, respectively. Density functional calculations showed that Te adatoms preferentially bind at the terrace sites of Pd, thereby suppressing undesired hydrogen evolution, whereas CO2 adsorption and activation occurred on the high index sites of Pd to produce CO.
- This article is part of the themed collections: Most popular 2018-2019 catalysis articles and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...