Selective photocatalytic hydroxylation and epoxidation reactions by an iron complex using water as the oxygen source†
Abstract
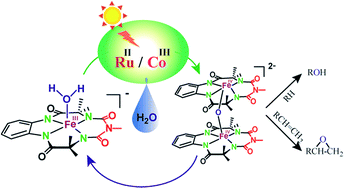
The iron complex [(bTAML)FeIII–OH2]− (1) selectively catalyses the photocatalytic hydroxylation and epoxidation reactions of alkanes and alkenes, respectively, using water as the oxygen-atom source. Upon the oxidation of unactivated alkanes, which included several substrates including natural products, hydroxylation was observed mostly at the 3° C–H bonds with 3° : 2° selectivity up to ∼100 : 1. When alkenes were used as the substrates, epoxides were predominantly formed with high yields. In the presence of H218O, more than 90% of the 18O-labelled oxygen atoms were incorporated into the hydroxylated and epoxide product indicating that water was the primary oxygen source. Mechanistic studies indicate the formation of an active [{(bTAML)FeIV}2-μ-oxo]2− (2) dimer from the starting complex 1via PCET. The subsequent disproportionation of 2 upon addition of substrate, leading to the formation of FeV(O), renders the high selectivity observed in these reactions.


 Please wait while we load your content...
Please wait while we load your content...