Nickel-catalyzed asymmetric hydrogenation of β-acylamino nitroolefins: an efficient approach to chiral amines†
Abstract
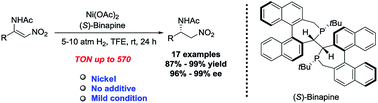
An efficient approach for synthesizing chiral β-amino nitroalkanes has been developed via the Ni-catalyzed asymmetric hydrogenation of challenging β-amino nitroolefins under mild conditions, affording the desired products in excellent yields and with high enantioselectivities. This protocol had good compatibility with the wide substrate scope and a range of functional groups. The synthesis of chiral β-amino nitroalkanes on a gram scale has also been achieved. In addition, the reaction mechanism was elucidated using a combined experimental and computational study, and it involved acetate-assisted heterolytic H2 cleavage followed by 1,4-hydride addition and protonation to achieve the nitroalkanes.


 Please wait while we load your content...
Please wait while we load your content...