Combining the catalytic enantioselective reaction of visible-light-generated radicals with a by-product utilization system†
Abstract
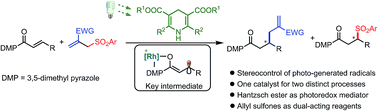
We report an unusual reaction design in which a chiral bis-cyclometalated rhodium(III) complex enables the stereocontrolled chemistry of photo-generated carbon-centered radicals and at the same time catalyzes an enantioselective sulfonyl radical addition to an alkene. Specifically, employing inexpensive and readily available Hantzsch esters as the photoredox mediator, Rh-coordinated prochiral radicals generated by a selective photoinduced single electron reduction are trapped by allyl sulfones in a highly stereocontrolled fashion, providing radical allylation products with up to 97% ee. The hereby formed fragmented sulfonyl radicals are utilized via an enantioselective radical addition to form chiral sulfones, which minimizes waste generation.


 Please wait while we load your content...
Please wait while we load your content...