From imine to amine: an unexpected left turn. Cis-β iron(ii) PNNP′ precatalysts for the asymmetric transfer hydrogenation of acetophenone†
Abstract
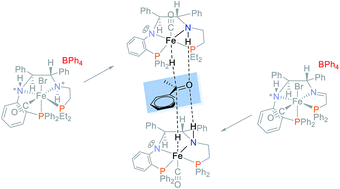
A novel PNN ligand bearing an orthophenylene group and a primary amine was synthesized with the aid of a palladium-catalyzed amination and reacted with phosphonium dimers [–PR2CH2CH(OH)–]2[Br]2 R = Et, iPr, Cy, Ph, xylyl, and o-Tol, and [Fe(OH2)6]2+ to produce a new series of cis-β iron(II) PNNP′ precatalysts cis-β-[Fe(Br)(CO)(PNNP′)]BPh4 as a pair of diastereomers. The more stable orthophenylene amido group was chosen to imitate and replace the enamido moiety of a highly active iron precatalyst for the asymmetric transfer hydrogenation (ATH) of ketones in an attempt to prevent its deactivation caused by reduction of the enamido group. This objective was partially achieved using the complex with a PEt2 group which catalyzed the transfer hydrogenation in isopropanol of 150 000 equivalents of acetophenone to racemic 1-phenylethanol. With a low acetophenone to catalyst ratio of 500 to 1, the catalytic activity was moderate and the enantiomeric excess (ee) of the product 1-phenylethanol ranged surprisingly from 94% (R) to 95% (S) depending on the nature of PR2 and whether the precatalyst contained an imine or amine donor. The amine precatalyst with a PEt2-group produced a more stable hydride species when activated, allowing the reaction mixture to be heated to 75 °C to obtain a TON of 8821 for acetophenone while retaining the high ee of 95% (S). The activation pathway in basic isopropanol (iPrOH) was studied for three precatalysts to elucidate that the cis-β precatalysts rearrange to form trans hydride complexes. The study suggests that the enantioselectivity of these complexes is determined by from which side of the PNNP′ plane the hydride transfer occurs.


 Please wait while we load your content...
Please wait while we load your content...