Rapid access to substituted 2-naphthyne intermediates via the benzannulation of halogenated silylalkynes†
Abstract
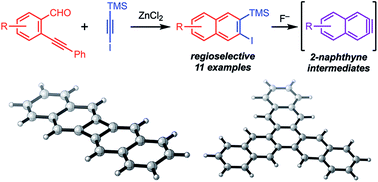
Aryne intermediates are versatile and important reactive intermediates for natural product and polymer synthesis. 2-Naphthynes are relatively unexplored because few methods provide precursors to these intermediates, especially for those bearing additional substituents. Here we report a general synthetic strategy to access 2-naphthyne precursors through an Asao-Yamamoto benzannulation of ortho-(phenylethynyl)benzaldehydes with halo-silylalkynes. This transformation provides 2-halo-3-silylnaphthalenes with complete regioselectivity. These naphthalene products undergo desilylation/dehalogenation in the presence of F− to generate the corresponding 2-naphthyne intermediate, as evidenced by furan trapping experiments. When these 2-naphthynes are generated in the presence of a copper catalyst, ortho-naphthalene oligomers, trinaphthalene, or binaphthalene products are formed selectively by varying the catalyst loading and reaction temperature. The efficiency, mild conditions, and versatility of the naphthalene products and naphthyne intermediates will provide efficient access to many new functional aromatic systems.


 Please wait while we load your content...
Please wait while we load your content...