Determination of protonation states of iminosugar–enzyme complexes using photoinduced electron transfer†
Abstract
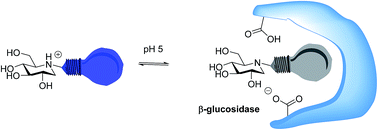
A series of N-alkylated analogues of 1-deoxynojirimycin containing a fluorescent 10-chloro-9-anthracene group in the N-alkyl substituent were prepared. The anthracene group acted as a reporting group for protonation at the nitrogen in the iminosugar because an unprotonated amine was found to quench fluorescence by photoinduced electron transfer. The new compounds were found to inhibit β-glucosidase from Phanerochaete chrysosporium and α-glucosidase from Aspergillus niger, with Ki values in the low micro- to nanomolar range. Fluorescence and inhibition versus pH studies of the β-glucosidase–iminosugar complexes revealed that the amino group in the inhibitor is unprotonated when bound, while one of the active site carboxylates is protonated.


 Please wait while we load your content...
Please wait while we load your content...