Double C–H bond activation of acetylene by atomic boron in forming aromatic cyclic-HBC2BH in solid neon†
Abstract
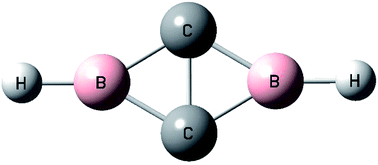
The organo-boron species formed from the reactions of boron atoms with acetylene in solid neon are investigated using matrix isolation infrared spectroscopy with isotopic substitutions as well as quantum chemical calculations. Besides the previously reported single C–H bond activation species, a cyclic-HBC2BH diboron species is formed via double C–H bond activation of acetylene. It is characterized to have a closed-shell singlet ground state with planar D2h symmetry. Bonding analysis indicates that it is a doubly aromatic species involving two delocalized σ electrons and two delocalized π electrons. This finding reveals the very first example of double C–H bond activation of acetylene in forming new organo-boron compounds.


 Please wait while we load your content...
Please wait while we load your content...