Synthetic control and empirical prediction of redox potentials for Co4O4 cubanes over a 1.4 V range: implications for catalyst design and evaluation of high-valent intermediates in water oxidation†
Abstract
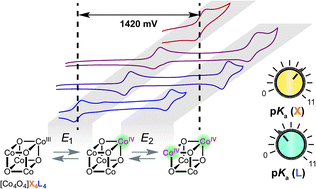
The oxo-cobalt cubane unit [Co4O4] is of interest as a homogeneous oxygen-evolution reaction (OER) catalyst, and as a functional mimic of heterogeneous cobalt oxide OER catalysts. The synthesis of several new cubanes allows evaluation of redox potentials for the [Co4O4] cluster, which are highly sensitive to the ligand environment and span a remarkable range of 1.42 V. The [CoIII4O4]4+/[CoIII3CoIVO4]5+ and [CoIII3CoIVO4]5+/[CoIII2CoIV2O4]6+ redox potentials are reliably predicted by the pKas of the ligands. Hydrogen bonding is also shown to significantly raise the redox potentials, by ∼500 mV. The potential-pKa correlation is used to evaluate the feasibility of various proposed OER catalytic intermediates, including high-valent Co-oxo species. The synthetic methods and structure–reactivity relationships developed by these studies should better guide the design of new cubane-based OER catalysts.


 Please wait while we load your content...
Please wait while we load your content...