Carbon dioxide hydrogenation catalysed by well-defined Mn(i) PNP pincer hydride complexes†
Abstract
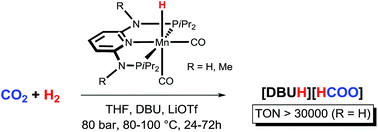
The catalytic reduction of carbon dioxide is of great interest for its potential as a hydrogen storage method and to use carbon dioxide as C-1 feedstock. In an effort to replace expensive noble metal-based catalysts with efficient and cheap earth-abundant counterparts, we report the first example of Mn(I)-catalysed hydrogenation of CO2 to HCOOH. The hydride Mn(I) catalyst [Mn(PNPNH-iPr)(H)(CO)2] showed higher stability and activity than its Fe(II) analogue. TONs up to 10 000 and quantitative yields were obtained after 24 h using DBU as the base at 80 °C and 80 bar total pressure. At catalyst loadings as low as 0.002 mol%, TONs greater than 30 000 could be achieved in the presence of LiOTf as the co-catalyst, which are among the highest activities reported for base-metal catalysed CO2 hydrogenations to date.


 Please wait while we load your content...
Please wait while we load your content...