Highly chemoselective ruthenium(ii)-catalyzed direct arylation of cyclic and N,N-dialkyl benzamides with aryl silanes†
Abstract
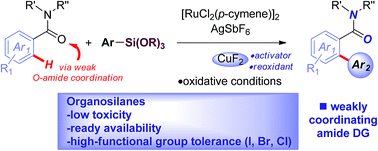
The ruthenium(II)-catalyzed oxidative cross-coupling of C(sp2)–H bonds with organosilanes has been accomplished for the first time. This novel protocol enlists challenging cyclic and N,N-dialkyl benzamides as weakly-coordinating substrates to achieve highly regioselective C(sp2)–H arylation as a proof-of-concept, taking advantage of the attractive features of organosilanes as coupling partners. This innovative method is characterized by very high chemoselectivity, installing halide functional groups (I, Br, Cl) that are incompatible with Ru(II)-carboxylate systems employing halides as cross-coupling partners, while obviating the need for sensitive organometallic reagents and cryogenic temperatures typical to the classic directed-ortho-metallation (DoM) techniques, employing benzamides to afford bioactive structural motifs.


 Please wait while we load your content...
Please wait while we load your content...