Racemic hemiacetals as oxygen-centered pronucleophiles triggering cascade 1,4-addition/Michael reaction through dynamic kinetic resolution under iminium catalysis. Development and mechanistic insights†
Abstract
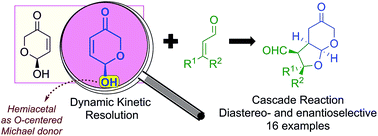
2-Hydroxydihydropyran-5-ones behave as excellent polyfunctional reagents able to react with enals through oxa-Michael/Michael process cascade under the combination of iminium and enamine catalysis. These racemic hemiacetalic compounds are used as unconventional O-pronucleophiles in the initial oxa-Michael reaction, also leading to the formation of a single stereoisomer under a dynamic kinetic resolution (DKR) process. Importantly, by using β-aryl or β-alkyl substituted α,β-unsaturated substrates as initial Michael acceptors either kinetically or thermodynamically controlled diastereoisomers were formed with high stereoselection through the careful selection of the reaction conditions. Finally, a complete experimental and computational study confirmed the initially proposed DKR process during the catalytic oxa-Michael/Michael cascade reaction and also explained the kinetic/thermodynamic pathway operating in each case.


 Please wait while we load your content...
Please wait while we load your content...