Fluorocarbene, fluoroolefin, and fluorocarbyne complexes of Rh†
Abstract
The manuscript reports the synthesis, characterization, and analysis of electronic structure in a series of complexes of small perfluorocarbon ligands with the (PNP)Rh fragment (where PNP is a diarylamido/bis(phosphine) pincer ligand). Reactions of (PNP)Rh(TBE) as the source of (PNP)Rh with CHF3 and C2HF5 produced perfluoroalkylidene complexes (PNP)Rh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 and (PNP)Rh
CF2 and (PNP)Rh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(F)(CF3). (PNP)Rh
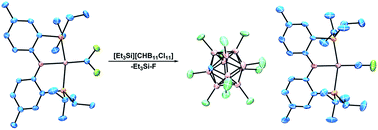
C(F)(CF3). (PNP)Rh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 could also be obtained via the reaction of (PNP)Rh(TBE) with Me3SiCF3/CsF, with an admixture of (PNP)Rh(C2F4), where TBE = tert-butylethylene. Abstraction of fluoride from these neutral (PNP)RhCxFy complexes was successful, although only abstraction from (PNP)Rh
CF2 could also be obtained via the reaction of (PNP)Rh(TBE) with Me3SiCF3/CsF, with an admixture of (PNP)Rh(C2F4), where TBE = tert-butylethylene. Abstraction of fluoride from these neutral (PNP)RhCxFy complexes was successful, although only abstraction from (PNP)Rh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 allowed unambiguous identification of the Rh product, [(PNP)Rh
CF2 allowed unambiguous identification of the Rh product, [(PNP)Rh![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CF]+. DFT computational studies allowed comparison of relative energies of (PNP)Rh(C2F4) and [(PNP)Rh(C2F3)]+ isomers as well as comparisons between the electronic structure of the
CF]+. DFT computational studies allowed comparison of relative energies of (PNP)Rh(C2F4) and [(PNP)Rh(C2F3)]+ isomers as well as comparisons between the electronic structure of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2, C2F4, and
CF2, C2F4, and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CF+ complexes and their hydrocarbon analogues.
CF+ complexes and their hydrocarbon analogues.


 Please wait while we load your content...
Please wait while we load your content...