Organocatalytic activation of isocyanides: N-heterocyclic carbene-catalyzed enaminone synthesis from ketones†
Abstract
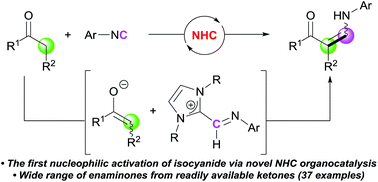
The first example of the use of an N-heterocyclic carbene (NHC) as an organocatalyst for the activation of isocyanides was demonstrated. On the basis of previous reports on the interaction between NHCs and isocyanides, we developed a catalytic cycle involving transient imidoyl intermediate. The reaction of ketones with isocyanides produced the corresponding enaminones with high efficiency. Control experiments suggested a novel role for the carbene in the activation of isocyanides, and a proton transfer process was found to be crucial for the generation of two activated species in the catalytic cycle. Various enaminones, some of which are not easily accessible by other methods, were synthesized in excellent yields. This study clearly demonstrates the potential of the nucleophilic activation of isocyanides in the expansion of their reactivity scope.


 Please wait while we load your content...
Please wait while we load your content...