Catalytic activity of catalase–silica nanoparticle hybrids: from ensemble to individual entity activity†
Abstract
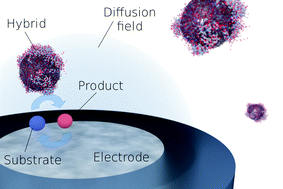
We demonstrate the electrochemical detection and characterization of individual nanoparticle–enzyme hybrids. Silica nanoparticles were functionalized with catalase enzyme and investigated spectroscopically and electrochemically. The catalytic activity of the hybrids towards hydrogen peroxide decomposition was comparable to the activity of a freely diffusing enzyme in solution, exhibiting a Michaelis–Menten constant of KM = 74 mM and a turnover number of kcat = 8 × 107 s−1 per NP. The fast turnover number of the hybrid further enabled the electrochemical detection of individual nanoparticle–enzyme hybrid via a novel method: the hydrogen peroxide substrate was generated at a microelectrode which enabled enzymatic activity exclusively within the diffusion layer of the electrode. The method is the first electrochemical approach for measuring hybrid nanoparticles, at the single entity level.


 Please wait while we load your content...
Please wait while we load your content...