Unexpected right-handed helical nanostructures co-assembled from l-phenylalanine derivatives and achiral bipyridines†
Abstract
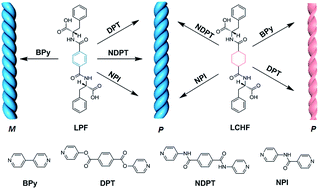
The construction of chiral supramolecular systems with desirable handedness is of great importance in materials science, chemistry, and biology since chiral nanostructures exhibit fascinating photophysical properties and unique biological effects. Herein, we report that achiral bipyridines can co-assemble with L-phenylalanine derivatives into unexpected right-handed helical nanostructures rather than a left-handed helix via intermolecular hydrogen bonding interactions formed between the pyridyl and carboxylic groups. This study opens up a route to develop chiral nanostructures with desirable handedness via the co-assembly of simple molecular building blocks and provides a straightforward insight into the chirality control of nanostructures in supramolecular systems.


 Please wait while we load your content...
Please wait while we load your content...