Synthesis of rhamnosylated arginine glycopeptides and determination of the glycosidic linkage in bacterial elongation factor P†
Abstract
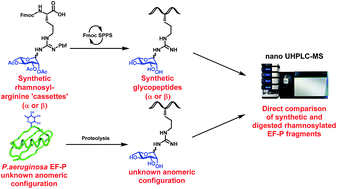
A new class of N-linked protein glycosylation – arginine rhamnosylation – has recently been discovered as a critical modification for the function of bacterial elongation factor P (EF-P). Herein, we describe the synthesis of suitably protected α- and β-rhamnosylated arginine amino acid “cassettes” that can be directly installed into rhamnosylated peptides. Preparation of a proteolytic fragment of Pseudomonas aeruginosa EF-P bearing both α- and β-rhamnosylated arginine enabled the unequivocal determination of the native glycosidic linkage to be α through 2D NMR and nano-UHPLC-tandem mass spectrometry studies.


 Please wait while we load your content...
Please wait while we load your content...