The interplay between fluorescence and phosphorescence with luminescent gold(i) and gold(iii) complexes bearing heterocyclic arylacetylide ligands†
Abstract
The photophysical properties of a series of gold(I) [LAu(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)] (L = PCy3 (1a–4a), RNC (5a), NHC (6a)) and gold(III) complexes [Au(C^N^C)(C
CR)] (L = PCy3 (1a–4a), RNC (5a), NHC (6a)) and gold(III) complexes [Au(C^N^C)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)] (1b–4b) bearing heterocyclic arylacetylide ligands with narrow band-gap are compared. The luminescence of both series are derived from an intraligand transition localized on the arylacetylide ligand (ππ*(C
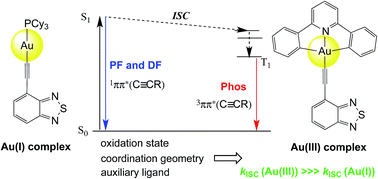
CR)] (1b–4b) bearing heterocyclic arylacetylide ligands with narrow band-gap are compared. The luminescence of both series are derived from an intraligand transition localized on the arylacetylide ligand (ππ*(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)) but 1a–3a displayed prompt fluorescence (τPF = 2.7–12.0 ns) while 1b–3b showed mainly phosphorescence (τPh = 104–205 μs). The experimentally determined intersystem crossing (ISC) rate constants (kISC) are on the order of 106 to 108 s−1 for the gold(I) series (1a–3a) but 1010 to 1011 s−1 for the gold(III) analogues (1b–3b). DFT/TDDFT calculations have been performed to help understand the difference in the kISC between the two series of complexes. Owing to the different oxidation states of the gold ion, the Au(I) complexes have linear coordination geometry while the Au(III) complexes are square planar. It was found from DFT/TDDFT calculations that due to this difference in coordination geometries, the energy gap between the singlet and triplet excited states (ΔEST) with effective spin–orbit coupling (SOC) for Au(I) systems is much larger than that for the Au(III) counterparts, thus resulting in the poor ISC efficiency for the former. Time-resolved spectroscopies revealed a minor contribution (<2.9%) of a long-lived delayed fluorescence (DF) (τDF = 4.6–12.5 μs) to the total fluorescence in 1a–3a. Attempts have been made to elucidate the mechanism for the origins of the DF: the dependence of the DF intensity with the power of excitation light reveals that triplet–triplet annihilation (TTA) is the most probable mechanism for the DF of 1a while germinate electron–hole pair (GP) recombination accounts for the DF of 2a in 77 K glassy solution (MeOH/EtOH = 4 : 1). Both 4a and 4b contain a BODIPY moiety at the acetylide ligand and display only 1IL(ππ*) fluorescence with negligible phosphorescence being observed. Computational analyses attributed this observation to the lack of low-lying triplet excited states that could have effective SOC with the S1 excited state.
CR)) but 1a–3a displayed prompt fluorescence (τPF = 2.7–12.0 ns) while 1b–3b showed mainly phosphorescence (τPh = 104–205 μs). The experimentally determined intersystem crossing (ISC) rate constants (kISC) are on the order of 106 to 108 s−1 for the gold(I) series (1a–3a) but 1010 to 1011 s−1 for the gold(III) analogues (1b–3b). DFT/TDDFT calculations have been performed to help understand the difference in the kISC between the two series of complexes. Owing to the different oxidation states of the gold ion, the Au(I) complexes have linear coordination geometry while the Au(III) complexes are square planar. It was found from DFT/TDDFT calculations that due to this difference in coordination geometries, the energy gap between the singlet and triplet excited states (ΔEST) with effective spin–orbit coupling (SOC) for Au(I) systems is much larger than that for the Au(III) counterparts, thus resulting in the poor ISC efficiency for the former. Time-resolved spectroscopies revealed a minor contribution (<2.9%) of a long-lived delayed fluorescence (DF) (τDF = 4.6–12.5 μs) to the total fluorescence in 1a–3a. Attempts have been made to elucidate the mechanism for the origins of the DF: the dependence of the DF intensity with the power of excitation light reveals that triplet–triplet annihilation (TTA) is the most probable mechanism for the DF of 1a while germinate electron–hole pair (GP) recombination accounts for the DF of 2a in 77 K glassy solution (MeOH/EtOH = 4 : 1). Both 4a and 4b contain a BODIPY moiety at the acetylide ligand and display only 1IL(ππ*) fluorescence with negligible phosphorescence being observed. Computational analyses attributed this observation to the lack of low-lying triplet excited states that could have effective SOC with the S1 excited state.

 Please wait while we load your content...
Please wait while we load your content...