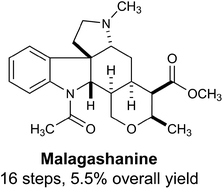
Total synthesis of malagashanine: a chloroquine potentiating indole alkaloid with unusual stereochemistry†
Abstract
The first total synthesis of malagashanine, a chloroquine potentiating indole alkaloid, is presented. A highly stereoselective cascade annulation reaction was developed to generate the tetracyclic core of the Malagasy alkaloids. This chemistry is likely to be broadly applicable to the synthesis of other members of this stereochemically unique family of natural products.

 Please wait while we load your content...
Please wait while we load your content...