A metal-mediated base pair that discriminates between the canonical pyrimidine nucleobases†
Abstract
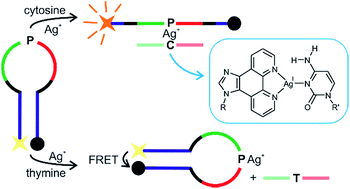
A nucleoside analogue comprising the ligand 1H-imidazo[4,5-f][1,10]phenanthroline (P) was applied to develop a molecular beacon capable of discriminating the canonical nucleobases cytosine and thymine. The beacon is based on the formation of a stable Ag+-mediated base pair between P and cytosine, whereas the presence of Ag+ strongly destabilizes nucleic acids comprising an artificial base pair between P and thymine. Metal-mediated base pair formation was investigated by temperature-dependent UV spectroscopy and CD spectroscopy and complemented by extensive DFT calculations. The molecular beacon significantly extends the application spectrum of nucleic acids with metal-mediated base pairs. It is of potential use in the detection of single-nucleotide polymorphisms.


 Please wait while we load your content...
Please wait while we load your content...